How suitable are secondary resources from the demolition and construction industry for the production of novel geopolymer construction materials?
The global production of high-quality building materials causes enormous resource consumption and releases considerable amounts of CO2, which counteracts the European and global climate protection targets. Accordingly, more resource- and climate-friendly building materials based on the upcycling of mineral residues should be increasingly used in the future. So-called geopolymers are a promising class of building materials. In the work presented here, geopolymer building materials are developed from processed mineral construction residues and their material parameters are investigated. By using processed demolition materials, raw material resources are recycled and landfill space is conserved at the same time. CO2 emissions are saved through a lower-energy manufacturing process.
1 Introduction and objectives
In 2019, 222 678 new construction projects were approved in Germany alone [1]. For the erection of newbuilds on this scale, in line with requirements, correspondingly large quantities of suitable construction materials, e.g. concrete, aerated concrete, sand-lime bricks or clay bricks, are needed. Common to all construction materials is that significant material and energy resources must be used for their production in thermal processing plants. During their thermal treatment at high temperatures, both because of the combustion of primary fuels and because of the decarbonization of the mineral input materials, corresponding quantities of CO2 are emitted.
To meet climate protection goals, key factors for the construction materials of the future are a lower energy consumption already during production, a reduction in CO2 emissions, a viable content of recycled materials and their return into the material cycle. In Germany alone, over 228 mill. t construction and demolition waste were produced in 2018 [2]. Recoverables have already been usefully recycled for many years. Metallic components like iron, steel and copper cables, wood and plastics are separated from inorganic wall building materials like plaster and mortar and returned to the material cycle. However, a large part of the inorganic demolition materials is sent to construction waste landfills, blocking valuable landfill space [3, 4].
The work presented here addresses a novel class of building materials, so-called geopolymers. These are cold-hardening materials that need comparatively low primary energy with correspondingly low CO2 emissions [5]. From the literature data, it is known that geopolymers have been synthesized on the basis of metakaolin, fly ash and blast furnace slags, thus saving primary energy and costs [6]. Fly-ash-based geopolymers are proving especially environmentally friendly in comparison. This is attributed primarily to the fact that no combustion or drying processes are necessary [6]. Going further with this environmental thinking, geopolymers have also been produced on the basis of broken bricks and concrete waste [8 – 12]. In the following, various typical demolition and waste materials from the construction sector such as broken bricks, brick grinding dust, mixed construction waste and concrete construction waste are tested to gauge their suitability as recycled raw materials for the production of novel geopolymer building materials. A specific objective of the tests is whether the above-mentioned waste materials are already suitable in their “pure form” as singular solid components for geopolymer production.
2 Materials and methods
2.1 Current situation
According to current knowledge, geopolymerization is a complex multiphase reaction process divided into three main steps:
1. The partial dissolution and dissolution of silicates and aluminosilicates e.g. in metakaolin or fly ash based on the break-up of Si-O-Si – or Si-O-Al – bonds in alkaline solution. In this process, tetrahedral silicate and aluminate monomers are formed in accordance with the following simplified reaction equation [13; 14]:
Al-Si-Mineral + NaOH (aq)⇥Na+ + Al(OH)4- + Si(OH)4⇥[1]
2. The accumulation phase in which the silicate and aluminate tetrahedra alternately link with each other as a result of condensation reactions:
⇥[2]
3. The interlinking in which the entire system is transformed into an inorganic three-dimensional network, the geopolymer [15 – 17].
⇥[3]
2.2 Materials
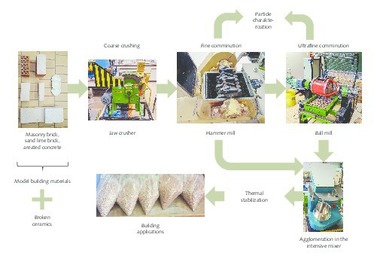
The waste materials used here are single-origin brick grinding dust (Fig. 1 a) and single-origin broken bricks (Fig. 1b, 1c), mixed construction waste (Fig. 1 d, e) and concrete construction waste (Fig. 1 f, g). While the brick grinding dust is already available in powder form, the waste fractions in lumps, that is broken brick, mixed and concrete construction rubble were first comminuted in a Bond ball mill and then screened to a particle size x ≤ 200 µm. Only the screen undersize was used for the subsequent tests on the production of geopolymers. In addition, a powder class F – fly ash from a coal-fired power station with the name Microsit 10 (Fig. 1 h) was selected as another waste material.
2.3 Test methods and sample preparation
2.3.1 X-ray fluorescence analysis
For chemical characterization of the different waste fractions, X-ray fluorescence analysis was conducted. For this purpose, an Axios XRF device from PANalytical was used, this being a wavelength dispersive sequency spectrometer (WDXRF), operated with a rhodium anode. For quantitative analysis of the waste materials, first the loss on ignition was determined at 950 °C over a time period of 2 h. In the following, the powder samples were mixed with di-lithium tetraborate (Li₂B₄O₇) and melted at 1070 °C in platinum crucibles. The obtained fused tablets were analysed with the above-mentioned XRF device and evaluated with the associated software.
2.3.2 X-ray diffraction analysis
The mineralogical phases of the waste materials were determined with an X-ray diffractometer (XRD). For this purpose, the powder samples were prepared in the so-called backloading process. For analysis of the samples, an X’Pert PRO X-ray diffractometer from PANalytical was used. The cathode ray tube of this diffractometer is operated with a copper anode. The generator was set at 40 mA at 45 kV. The diffraction diagrams obtained were evaluated with the help of a database and the mineral composition determined from this.
2.3.3 Production of geopolymer bricks from waste materials
For the production of the geopolymer bricks, an alkaline activation solution was produced from a liquid sodium silicate waterglass of the type “Betol 39 t” supplied by Woellner-Werke, Ludwigshafen. During constant stirring, solid NaOH pellets were slowly added until the solution consisted of 85.46 wt% liquid sodium silicate and 14.54 wt% sodium hydroxide. The solution was stirred constantly over a period of 24 hours.
For production of the geopolymer bricks, 1 kg activator solution was added to 3 kg prepared waste material powder. In this test series, the geopolymer bricks were produced exclusively from 100 wt% of the prepared single-source waste material aggregate. The test batches were homogenized in a laboratory mixer over a period of 15 min in each case, at a rotational speed of 250 rpm. The homogeneous paste was immediately filled into silicon moulds and any air bubbles introduced in this process driven out over a period of 5 min on a vibratory plate. For hardening, the binder construction material samples were stored in defined conditions, namely over a period of 48 hours in each case and, protected against drying out with PE film, at a temperature of 85 °C in a drying cabinet.
After the hardening process, the geopolymer test pieces were demoulded, and then sawn and ground to suitable test specimens. The test specimens for the compressive strength tests were prepared with an edge length of (L x W x H) 40 mm x 40 mm x 40 mm, those for the three-point bending tests with an edge length of (L x W x H) 160 mm x 40 mm x 40 mm and test plates for testing thermal conductivity with the dimensions (L x W x H) 100 mm x 100 mm x 25 mm. All the specimens that came into contact with water during sawing and grinding were dried to constant weight in the drying cabinet prior to measurement of the material characteristics and then stored in a desiccator until they could be measured.
2.3.4 Material tests
Determination of compressive and flexural strength was performed with reference to DIN EN 196 Part 1 [18]. For the compressive strength testing, the pressure was generated on the test surface at a rate of 1.5 N (mm2 s)-1, and for the bending strength test at a rate of 0.05 kN s-1.
2.3.5 Determination of the density and thermal conductivity
For these tests, the dry plate-like test specimens cooled to room temperature are weighed and the outer specimen dimensions measured. The density of the test specimens is measured in accordance with the formula “density = mass / volume. The thermal conductivity λ10,tr. is measured with reference to test standard DIN EN ISO8302:1991, using the so-called plate method [19]. Here the specimen to be measured is fixed between a heated and cooling plate and thermal conductivity measured at 15 °C, 20 °C and 35 °C specimen mean temperature. Several sensors constantly determine the actual temperatures of the specimen surfaces. Moreover, the electric power and current needed to maintain the temperatures are also monitored. The thermal conductivity is calculated as a function of the respective specimen mean temperature with Equation (4) below, the surface A of the specimen and layer thickness d also being taken into account:
⇥[4]
Then, based on linear regression, the thermal conductivity λ10,tr. is read at the specimen mean temperature of 10 °C.
3 Results
3.1 Chemical analysis of the waste materials
The chemical composition of the waste materials was analysed by means of X-ray fluorescence analysis (XRF). The results are listed in Table 1. The loss on ignition was determined at 950 °C in each case.
From the analysis, it can be seen that all analysed waste materials contain between 52.4 and 63.4 wt% SiO2. The Al2O3 content of the brick grinding dust, broken bricks and fly ash ranges between 17.9 and 25.2 wt%. The Al2O3 content in the mixed and concrete construction waste, on the other hand, measures only 5.3 and 3.1 wt% respectively. For the CaO content, it is the other way round. Here the mixed and concrete construction waste materials have a comparatively high content of 13.4 and 17.9 wt%, respectively, while the brick grinding dust contains only 5.4 wt%, the broken bricks only 8.6 wt% and the fly ash only 4.2 wt% CaO. All the waste materials contain a relatively low amount of trace constituents.
From Equations 1 to 3, it can be seen that the silicate and aluminate contents in the waste materials play a significant part in the formation of the geopolymers as these provide the structure-forming molecules/units. As the SiO2 content in all waste materials is roughly in the same order, and in the brick grinding dust, broken bricks and fly ash, the Al2O3 content is also in a similar order, similar geopolymer reactions can be expected for these waste materials. As the mixed and concrete construction waste, however, exhibits a lower Al2O3 content, with these waste materials a different polymerization results, leading to correspondingly different material properties [20 – 22]. It can also be expected that the trace elements contained in the waste materials are integrated largely as poorly soluble components in the forming geopolymer structure [21].
3.2 Mineralogical analysis of the waste materials
The results of the mineral analysis below correlate with the results of the chemical analysis. From the mineralogical analysis (XRD) it can be seen that the brick grinding dust, the diffractogram of which is shown as an example for all measurements in Fig. 3, contains the minerals quartzite, haematite, gehlenite, albite and microcline. In the broken bricks, on account of its same origin, the same minerals, namely quartzite, haematite, gehlenite, albite and microcline, are contained. In addition, muscovite and sodium-alumina silicates are detected. The mixed construction waste contains quartzite, calcite, dolomite and sanidine. Other mineralogical phases that would be expected cannot be detected on account of their concentration being too small. The concrete waste contains the mineral phases quartzite, haematite, gehlenite, albite and microcline. The analysis of the fly ash shows that this contains the minerals quartzite, haematite, gehlenite, albite and microcline.
3.3 Determination of the compressive strength
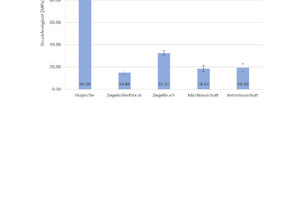
For the determination of the compressive strength, 15 geopolymer specimens for each composition are measured. A mean value and the standard deviations calculated from the results (Fig. 4). Fig. 4 shows that geopolymers prepared with pure fly ash (100 wt%) reach the highest compressive strengths at 96.6 MPa. The poorest compressive strengths at 14.9 MPa are exhibited by the geopolymers prepared with pure brick grinding dust (100 wt%). If the brick grinding dust is substituted with ground broken bricks (100 wt%), geopolymer compressive strengths of 32.31 MPa are obtained. The geopolymers produced with pure mixed construction waste (100 wt%) and pure concrete construction waste (100 wt%) exhibit compressive strengths of 18.7 MPa and 19.3 MPa.
3.4 Determination of the flexural strength
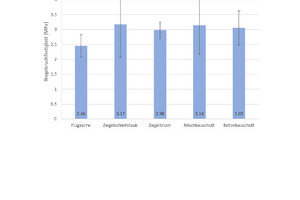
For determination of the mean flexural strength, six specimens of each type of geopolymer were tested and the mean values and standard deviations calculated from the tests (Fig. 5), it can be seen that the flexural strength of all types of geopolymers is in the same order. The geopolymers produced with pure fly ash (100 wt%) attain the lowest values at 2.5 MPa; the geopolymers with pure brick grinding dust (100 wt%) demonstrate the highest flexural strength, reaching 3.2 MPa.
3.5 Determination of the density and thermal conductivity
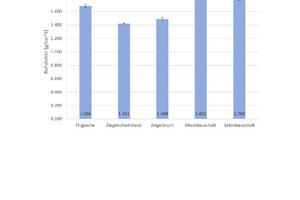
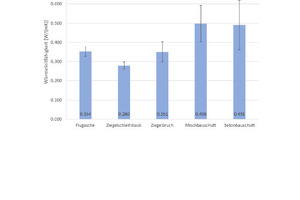
Fig. 6 shows the results of the density determination and Fig. 7 the results of the measurement of the thermal conductivity λ10,tr., with the associated standard deviations. For the geopolymer bricks with brick grinding dust and finely ground broken bricks, the tests show a low density of 1.4 g/cm3 and 1.5 g/cm3, respectively, and correlating with this, the lowest thermal conductivity λ10,tr. in this test series at 0.28 W/(mK) and 0.35 W/(mK). The geopolymer bricks with mixed and concrete construction waste show higher densities of 1.8 g/cm3 and, associated with this, much higher thermal conductivity λ10,tr. of 0.49 W/(mK). The geopolymer bricks prepared from fly ash have a mean density of 1.7 g/cm3 and correspondingly demonstrate a mean thermal conductivity λ10,tr. of 0.35 W/(mK).
4 Conclusions
The results presented here show that it is possible to produce new construction materials on the basis of geopolymer materials from single-source prepared construction waste. On account of different pre-treatment and chemical compositions of the construction waste materials used, the material results for the geopolymer types differ correspondingly widely. With the use of fly ash, high-strength geopolymers can be produced. This effect can be explained by the fact that the fly ash, depending on its origin, has comparatively more activated silicate and aluminate groups, which on account of the activation solution used are broken up slightly and available as reaction partners for the geopolymerization. In contrast with the fly ash geopolymer, the geopolymers prepared with the other construction waste materials exhibit comparatively high flexural strengths. With regard to the geopolymer densities and associated thermal conductivity λ10,tr., the geopolymers made from brick grinding dust and broken brick exhibit much lower and therefore better values with regard to thermal insulation properties than the fly-ash-based geopolymers. This effect can be attributed to the higher microporosity of the brick materials.
Overall, it is possible to produce intact geopolymer bricks from all the single-source mineral construction waste materials used here. This is even true of the mixed and concrete construction waste that possibly contain less optimally accessible silicate and aluminate groups. Thanks to this investigation, the main material parameters of the tested single-source waste material geopolymers are known. In further research, mixes of these waste materials can be prepared and, from these, geopolymer bricks with tailored properties produced. Pore formation with organic and inorganic poreformers for the production of high-thermal insulation geopolymers and geopolymer foams is another option. With the material recycling of processed construction waste to new substitute construction materials, it is possible to close previously open material cycles, save raw material resources and valuable landfill volume as well as to cut unnecessary CO2 emissions. It can be expected that at the end of their use, used geopolymer construction materials can be comminuted and ground for recycling into the production of new geopolymer construction.
Literatur • Literature
[1] Statistisches Bundesamt: Bautätigkeit – Fachserie 5 Reihe 1 – 2019 (2020)
[2] Statistisches Bundesamt: Abfallbilanz (Abfallaufkommen/-verbleib, Abfallintensität, Abfallaufkommen nach Wirtschaftszweigen) – 2018
[3] Fehn, T.; Teipel, U.: Werkstoffliche Aufbereitung von Wärmedämmverbundsystemen. In: Chemie Ingenieur Technik 92 (2020), Heft 4, S. 431-440. https://doi.org/10.1002/cite.201900124
[4] Fehn, T.; Kugler, F.; Tübke, B. et al.: Charakterisierung und Störstoffanalyse von rückgewonnenen Stoffströmen aus Wärmedämmverbundsystemen. In: Chemie Ingenieur Technik 93 (2021), Heft 5, S. 771-780. https://doi.org/10.1002/cite.202000215
[5] Provis, J.L.: Alkali-activated materials. In: Cement and Concrete Research 114 (2018), S. 40-48. https://doi.org/10.1016/j.cemconres.2017.02.009
[6] Izquierdo, M.; Querol, X.; Davidovits, J. et al.: Coal fly ash-slag-based geopolymers: microstructure and metal leaching. In: Journal of hazardous materials, Vol. 166 (2009), Iss. 1, pp. 561-566. https://doi.org/10.1016/j.jhazmat.2008.11.063
[7] Weil, M.; Buchwald, A.; Dombrowski-Daube, K.: How to Assess the Environmental Sustainability of Geopolymers? A Live Cycle Perspective. In: Advances in Science and Technology 69 (2010), S. 186-191. https://doi.org/10.4028/www.scientific.net/AST.69.186
[8] Zawrah, M.F.; Gado, R.A.; Feltin, N. et al.: Recycling and utilization assessment of waste fired clay bricks (Grog) with granulated blast-furnace slag for geopolymer production. In: Process Safety and Environmental Protection 103 (2016), S. 237-251. https://doi.org/10.1016/j.psep.2016.08.001
[9] Mohajerani, A.; Suter, D.; Jeffrey-Bailey, T. et al.: Recycling waste materials in geopolymer concrete. In: Clean Technologies and Environmental Policy 21 (2019), Heft 3, S. 493-515. https://doi.org/10.1007/s10098-018-01660-2
[10] Fořt, J.; Vejmelková, E.; Koňáková, D. et al.: Application of waste brick powder in alkali activated aluminosilicates: Functional and environmental aspects. In: Journal of Cleaner Production 194 (2018), S. 714-725. https://doi.org/10.1016/j.jclepro.2018.05.181
[11] Kugler, F.; Fehn, T.; Sandner, M. et al.: Microstructural and mechanical properties of geopolymers based on brick scrap and fly ash. In: International Journal of Ceramic Engineering & Science (2022). https://doi.org/10.1002/ces2.10120
[12] Kugler, F.; Karsdorf, R.; Fehn, T. et al.: Poster T1.S2 Felix.Kugler Suitability of construction and demolition residuals for the production of novel geopolymer building materials – slide 1, Ausgabe 2021
[13] J. Davidovits: Geopolymers – Inorganic polymeric new materials. In: Journal of Thermal Analysis 1991 (1991), Heft 37, S. 1633-1656
[14] Xu, H.; van Deventer, J.S.J.: The geopolymerisation of alumino-silicate minerals. In: International Journal of Mineral Processing 59 (2000), S. 247-266
[15] Mo, B.-h.; Zhu, H.; Cui, X.-m. et al.: Effect of curing temperature on geopolymerization of metakaolin-based geopolymers. In: Applied Clay Science 99 (2014), S. 144-148. https://doi.org/10.1016/j.clay.2014.06.024
[16] Silva, P. de; Sagoe-Crenstil, K.; Sirivivatnanon, V.: Kinetics of geopolymerization: Role of Al2O3 and SiO2. In: Cement and Concrete Research 37 (2007), Heft 4, S. 512-518. https://doi.org/10.1016/j.cemconres.2007.01.003
[17] Provis, J.L.; van Deventer, J.S.J.: Geopolymerisation kinetics. 1. In situ energy-dispersive X-ray diffractometry. In: Chemical Engineering Science 62 (2007), Heft 9, S. 2309-2317. https://doi.org/10.1016/j.ces.2007.01.027
[18] DIN EN 196-1:2016-11, Prüfverfahren für Zement_-Teil_1: Bestimmung der Festigkeit; Deutsche Fassung EN_196-1:2016
[19] DIN EN ISO 8302:1991-08, Wärmeschutz; Bestimmung des stationären Wärmedurchlasswiderstandes und verwandter Eigenschaften; Verfahren mit dem Plattengerät (1991)
[20] Granizo, M.L.; Alonso, S.; Blanco-Varela, M.T. et al.: Alkaline Activation of Metakaolin: Effect of Calcium Hydroxide in the Products of Reaction. In: Journal of the American Ceramic Siciety (2002), Heft 85, S. 225-231
[21] van Deventer, J.S.J.; Provis, J.L.; Duxson, P. et al.: Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. In: Journal of hazardous materials, Vol. 139 (2007), Iss. 3, pp. 506-513. https://doi.org/10.1016/j.jhazmat.2006.02.044
[22] Christian Ka-Bik Yip: The role of Calcium in Geopolymerisation. PhD thesis, Department of Chemical and Biomolecular Engineerung, The University of Melbourne, Melbourne, 2004
Author:
M. Eng. Felix Kugler
TH Nuremberg, Materials Engineering, Nuremberg/Germany
Felix Kugler studied Materials Engineering and New Materials, Nano and Production Engineering at the Nuremberg University of Applied Sciences. Since 2018, he has been working as a research assistant at the Nuremberg University of Technology in the research group of Prof. Dr. Wolfgang Krcmar in the field of energy-efficient materials development. In collaboration with Prof. Dr. Ulrich Teipel and the University of Ulm, he is working towards his doctorate in the topic area of "Processing of mineral secondary raw materials".
Further authors:
Thomas Fehn M.Sc.,
Research assistant in the research group for "Particle Technology and Raw Material Innovation" (FPR) at the Technical University of Nuremberg
Maximilian Harder, Bachelor's student at Nuremberg University of Applied Sciences
Prof. Dr. rer. nat. Wolfgang Krcmar, Chairman of the Scientific Board and Spokesman of the Efficiency Research Department at the Nuremberg Energy Campus of the Nuremberg University of Applied Sciences
Prof. Dr.-Ing. Ulrich Teipel, Head of the Fraunhofer Research Group for "Particle Technology and Raw Material Innovation" (FPR) at Nuremberg University of Applied Sciences